Introduction
In recent years, meteorological disasters, including
droughts and severe winter weather, have occurred frequently in the
world. A number of diseases exhibit seasonal patterns in their
occurrence. Weather changes may effects cardiovascular, infectious,
respiratory, gastrointestinal and other diseases. Extreme climates
seriously affect health and have resulted in the increased
morbidity of a number of diseases, including gastrointestinal
bleeding (1), which exhibit a
higher frequency in the winter than in the summer.
Peptic ulcer bleeding (PUB) is the most common cause
of upper gastrointestinal bleeding, including gastric ulcers (GU)
and duodenal ulcers(DU). Excessive acid secretion is discovered in
some patients but may not the main factor in most ulcers. The major
etiologic factors in chronic peptic ulcer are mainly caused by
Helicobacter pylori (H. pylori) infection and nonsteroidal
anti-inflammatory drugs (NSAIDs). Certain studies have indicated
that the incidence of peptic ulcers in cold climates is
significantly higher than that in hot climates (2,3). A
previous study has additionally found that the incidence of PUB is
inversely proportional to the temperature and correlated with the
degree of temperature variation (3). Cold climate and rapid climate change
may induce PUB (2,3), but little is known regarding its
specific pathogenesis. Gastric mucosal damage and barrier factors
were therefore examined in extreme climates to clarify the
pathogenesis of PUB under extreme climate conditions.
Patients and Methods
Patients
The study was conducted with the approval of the
Ethics Committee of the First Affiliated Hospital of Nanchang
University (Nanchang, China). A total of 176 patients with active
peptic ulcer with or without bleeding who were undergoing
endoscopic examinations at the First Affiliated Hospital of
Nanchang University during periods of extreme hot or cold climate
were included in the study. The extreme hot climate period was
between July and September 2009, when the average temperature was
>30°C, and the extreme cold climate period was between December
2009 and February 2010, when the average temperature was <10°C.
The patient demographics are summarized in Table I. Patients were eligible for
inclusion in the present study if they met the following criteria:
i) Age >18 years; ii) resident of Jiangxi province; and iii)
presenting with active peptic ulcer. The active peptic ulcer had to
be in conformity with one of the following conditions: Continuity
of ≥5 mm, multiple ulcers or actively bleeding ulcer. The exclusion
criteria were as follows: i) Serious organ diseases of important
organs, including the heart, lung, liver, kidney and brain; ii)
pregnant or lactating females; iii) patients with mental health
problems and patients who are unable to provide a medical history;
and iv) patients who have received treatment with proton pump
inhibitors (PPIs) or other gastroprotective agents within the last
two weeks. The subjects were divided into high and low bleeding
risk groups according to their histories of PUB or observations of
hemorrhages upon endoscopic examination.
 | Table IPatient demographics according to
bleeding risk group and extreme climate conditions. |
Table I
Patient demographics according to
bleeding risk group and extreme climate conditions.
| High bleeding risk
group | Low bleeding risk
group |
|---|
|
|
|
|---|
| Climate
condition | Male (n) | Female (n) | Average age
(years) | Male (n) | Female (n) | Average age
(years) |
|---|
| Hot, >30°C | 23 | 14 | 40.51±15.73 | 23 | 14 | 41.97±14.92 |
| Cold, <10°C | 31 | 20 | 40.24±13.33 | 31 | 20 | 41.59±11.66 |
Specimen collection and processing
Gastric biopsies were collected from the patients
with informed consent by endoscopy. Two specimens were collected
each from the antrum and the gastric body. At the same time, 10 ml
gastric juice was collected and the pH value of the gastric juice
was measured precisely on-site. Biopsy specimens were fixed with
10% buffered formalin and embedded in paraffin. Sections (4 μm
thick) were cut from the wax blocks. Conventional hematoxylin and
eosin staining was used to exclude malignant ulcers. Periodic
acid-Schiff staining was used to measure the gastric mucus
thickness.
Detection of Helicobacter pylori
infection
H. pylori infection was detected using
modified Giemsa staining. The paraffin-embedded sections were
conventionally deparaffinized in xylene twice for 10 min,
rehydrated through a graded ethanol series to distilled water and
washed three times with distilled water. The sections were then
stained for 30 min with 2% Giemsa dye, washed with running water,
polarized twice with 95% alcohol, rapidly dehydrated twice with
100% alcohol, aired naturally and sealed with neutral gum. H.
pylori infection was identified by the presence of H.
pylori on the gastric antrum or body mucosa with an optical
microscope (Nikon 55i; Nikon Corporation, Tokyo, Japan) under high
magnification.
Immunohistochemistry
The expression of heat shock protein (HSP) 70,
occludin, nitric oxide synthase (NOS), epidermal growth factor
(EGF) and EGF receptor (EGFR) proteins in the epithelial cells of
the gastric mucous membrane was detected by immunohistochemistry.
Slides were deparaffinized in xylene twice for 10 min and
rehydrated through a graded ethanol series to distilled water,
prior to incubation for 8 min with a 3% hydrogen
peroxidase-methanol solution to inhibit endogenous peroxidase
activity. The slides were heated in 0.01 mol/l citrate buffer (pH
6.0) in a microwave oven for 15 min on high power for antigen
retrieval. The slides were then removed from the microwave oven and
cooled at room temperature.
The sections were incubated at 4°C overnight in a
humidified chamber with anti-occludin (Abcam, Cambridge, MA, USA),
anti-EGF (Abcam), anti-EGFR (Santa Cruz Biotechnology, Inc., Santa
Cruz, CA, USA), anti-HSP70 (Santa Cruz Biotechnology, Inc.) or
anti-NOS (Santa Cruz Biotechnology, Inc.) antibodies, each of which
was diluted to 1:400 in blocking solution. The sections were rinsed
in phosphate-buffered saline (PBS) and incubated for 30 min with
biotinylated secondary antibody (poly-peroxidase anti-mouse/rabbit
immunoglobulin G; Zymed, San Francisco, CA, USA). The sections were
then washed in PBS and incubated for 30 min at 37°C.
3,3′-Diaminobenzidine was used as the chromogen. The slides were
counterstained for 3 min with hematoxylin solution. Specimens with
known positive expression were used as positive controls for each
lesion, whereas the primary antibody was replaced with PBS to serve
as a negative control.
The intensity of protein expression was graded as
follows: 0, no staining; 1, weak staining; 2, moderate staining;
and 3, strong staining. The percentage of positive cells was
evaluated as follows: 0, ≤5.0%; 1, 5.1–25.0%; 2, 25.1–50%; 3,
50.1–75%; and 4, >75%. The degree of positive staining was
obtained by multiplying the intensity and the area of expression
for classification as follows: Undetectable (-), score of 0–2; mild
(+), score of 3–5; moderate (++), score of 6–8; and marked (+++),
score of 9–12. The (−) and (+) classifications were considered to
be negative results, whereas (++) and (+++) were considered to be
positive results for protein expression.
Statistical analysis
The SPSS 16.0 statistical software package (SPSS,
Inc., Chicago, IL, USA) was used for statistical processing.
Continuous data are expressed as the mean ± standard deviation and
were compared by analysis of variance. Categorical variables were
analyzed using the Mann-Whitney U test. All tests of significance
were two-sided. A difference with a value of P<0.05 was
considered statistically significant.
Results
Factors damaging the gastric mucosa
Level of gastric acid
The pH value of the gastric juice was lower in the
high bleeding risk group (1.93±1.04) than that in the low bleeding
risk group (2.05±1.27) in the extreme hot climate, but the
difference was not statistically significant. This was consistent
with the results for the extreme cold climate, in which no
significant difference was identified in the pH values of the
gastric juice between the high (1.00±0.81) and low (1.35±0.93)
bleeding risk groups (Table
II).
 | Table IIpH value and Helicobacter
pylori infection rate of the gastric antrum in the different
groups. |
Table II
pH value and Helicobacter
pylori infection rate of the gastric antrum in the different
groups.
| Group | pH value | H. pylori
infection rate (%) |
|---|
| Extreme hot |
| High bleeding
risk | 1.93±1.04a | 75.68 |
| Low bleeding
risk | 2.05±1.27 | 83.78 |
| Extreme cold |
| High bleeding
risk | 1.00±0.81a | 86.27 |
| Low bleeding
risk | 1.35±0.93 | 74.51 |
H. pylori infection
H. pylori predominantly exist in the mucosal
surface, mucus and gland cavity. Under high magnification
microscopy, H. pylori can be observed as spiral, comma or
seagull shapes. In the extreme hot climate, the overall rate of
H. pylori infection was 79.73% (59/74). No significant
difference was identified between the high (75.68%, 28/37) and low
(83.78%, 31/37) bleeding risk groups. In the extreme cold climate,
the overall rate of H. pylori infection was 80.39% (82/102).
No significant difference was identified in the rate of H.
pylori infection between the high (86.27%, 44/51) and low
(74.51%, 38/51) bleeding risk groups (Table II).
Barrier factors of the gastric
mucosa
In the extreme hot climate, no significant
differences were identified in the mucus thickness of the gastric
antrum and body between the high and low bleeding risk groups. The
protein expression levels of HSP70, occludin, NOS, EGF and EGFR
were not significantly different between the high and low bleeding
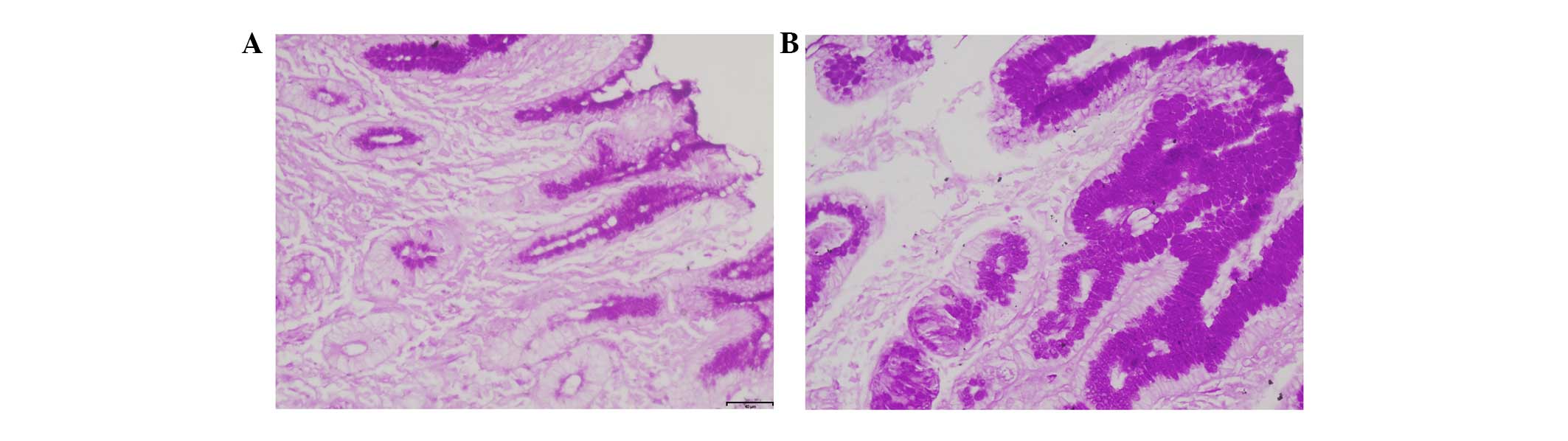
risk groups. In the extreme cold climate, the mucus thickness of
the gastric antrum was significantly lower in the high bleeding
risk group (4.81±1.59 μm) than that in the low bleeding risk group
(5.62±1.88 μm) (Fig. 1). The
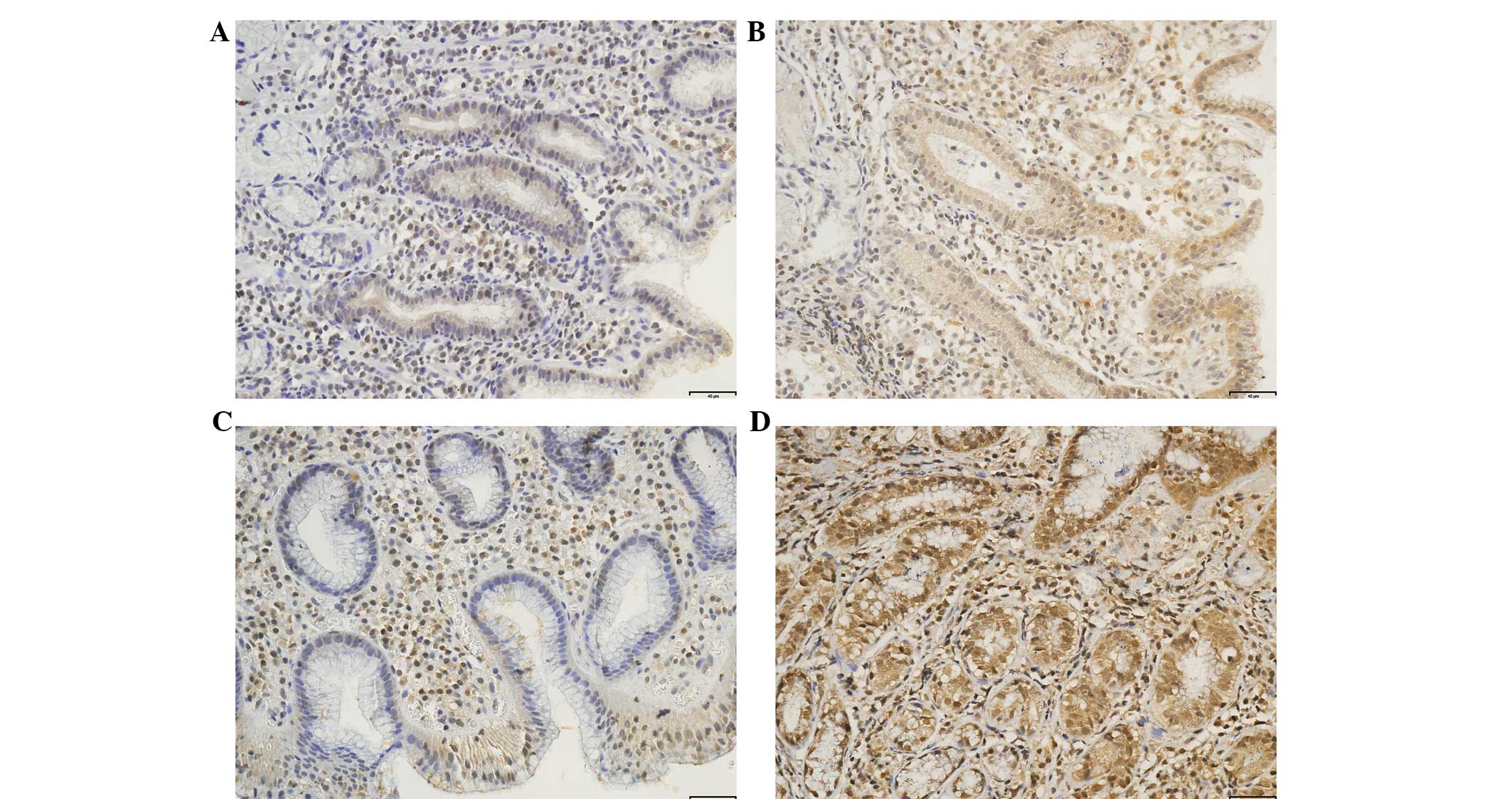
expression of HSP70 in the high bleeding risk group was
significantly lower than that in the low bleeding risk group
(Fig. 2 and Table III). No significant differences
were identified in the mucus thickness of the gastric body or the
expression of the occludin, NOS, EGF and EGFR proteins between the
high and low bleeding risk groups.
 | Table IIIExpression of HSP70 according to
patient group. |
Table III
Expression of HSP70 according to
patient group.
| | HSP70 protein
expression (gastric antrum) | HSP70 protein
expression (gastric body) |
|---|
| |
|
|
|---|
| Group | N | − (n) | + (n) | ++ (n) | +++ (n) | PR (%) | P-value | − (n) | + (n) | ++ (n) | +++ (n) | PR (%) | P-value |
|---|
| Extreme hot |
| High bleeding
risk | 37 | 2 | 4 | 11 | 20 | 83.8 | 0.923a | 1 | 4 | 11 | 21 | 86.5 | <0.001a |
| Low bleeding
risk | 37 | 2 | 5 | 9 | 21 | 81.1 | <0.001b | 0 | 4 | 10 | 23 | 89.1 | <0.001b |
| Extreme cold |
| High bleeding
risk | 51 | 25 | 15 | 3 | 8 | 21.6 | <0.001c | 11 | 31 | 6 | 3 | 17.6 | <0.001c |
| Low bleeding
risk | 51 | 8 | 18 | 20 | 5 | 74.5 | 0.002d | 5 | 24 | 16 | 6 | 43.1 | 0.005d |
The associations between climate and factors
associated with bleeding risk group were then examined. The pH of
the gastric acid, the mucus thickness of the gastric antrum, the
expression of the occludin and NOS proteins in the gastric antrum
(Fig. 3) and the expression of the
HSP70 protein in the gastric antrum and body were significantly
lower in the extreme cold climate than those in the hot climate. No
significant differences were identified in the mucus thickness of
the gastric body and the expression of the EGF and EGFR proteins in
the gastric antrum and body between the extreme cold and hot
climates.
Discussion
Acute upper gastrointestinal bleeding is a frequent
and life-threatening medical emergency, and its most common
etiological factor is PUB. Gastric mucosal damage and barrier
factors play important roles in the development of peptic ulcers.
Peptic ulcers are caused by defects in the gastro-duodenal mucosal
barrier that result from epithelial cell damage, which is evoked by
caustic agents, including gastric acid and pepsin (4). In general, PUB is induced by the
noxious effects of damaging factors (such as acid, pepsin and H.
pylori infection) prevailing over the barrier factors of the
gastro-duodenal mucosa (5).
According to Schwartz’s aphorism, ‘no acid, no ulcer’, gastric acid
is the crucial aggressive factor in PUB (6).
Since the development of PPIs, the incidence of PUB
has decreased (7). Furthermore,
according to a previous report (8), infusion treatment with a high dose of
PPI could reduce the bleeding recurrence rate, requirement for
surgery, length of hospital stay and mortality rate. Peptic ulcer
disease (PUD) can be effectively treated by suppressing acid, which
strongly suggests that the disease is largely caused by gastric
acid hypersecretion. In the present study, no significant
differences were observed in the pH values of the gastric juice
between the high and low bleeding risk groups in the extreme hot or
cold climates; however, it was observed that the pH value in the
high bleeding risk group was significantly higher in the extreme
cold climate than that in the extreme hot climate. This result may
explain why there was a higher incidence of peptic ulcers in the
cold climate than in the hot climate.
The discovery of H. pylori shifted the view
that PUD is simply an acid homeostasis disorder. Considerable
research has established that H. pylori infection is closely
associated with PUD (9). In the
present study, no significant difference was identified in the
H. pylori infection rate between the high or low bleeding
risk groups in the extreme hot or cold climates. These results were
consistent with those of Henriksson et al (10), which revealed no difference in the
prevalence of H. pylori infections in different climates.
Certain previous studies, however, have reported that the
prevalence of H. pylori infection was slightly lower in
patients with peptic ulcer complications than that in patients with
uncomplicated ulcer disease (11,12).
H. pylori infection and the use of nonsteroidal
anti-inflammatory drugs (NSAIDs) are independent risk factors for
PUB (13). Eradication of H.
pylori can significantly reduce the risk of recurrent peptic
ulcer and its complications (14).
Further studies are required to determine whether H. pylori
infection is one of the pathogenic factors for PUB in extreme
climates.
Impaired defensive function of the gastro-duodenal
mucosa is another important pathogenic factor of PUD. The
gastro-duodenal mucosal barrier includes at least three levels. The
first level of this defense system comprises the factors that are
secreted into the lumen, including mucus, bicarbonates and
immunoglobulins. The second level consists of the gastric
epithelia. The impermeability of the gastro-duodenal epithelium
makes it particularly resistant to the passive diffusion of acids
or irritants. In addition, the gastro-duodenal epithelium is
capable of rapid self-repair. The third level of the
gastro-duodenal mucosal barrier is the mucosal microcirculation.
Mucosal blood flow is critical for damage limitation and repair
facilitation. The tight junction protein occludin plays an
important role in the barrier function of the mucosa and acts to
effectively prevent the inverse dispersion of hydrogen ions into
the gastro-duodenal mucus. No significant difference was identified
in the expression level of occludin between the low and high
bleeding risk groups in the present study.
NO is a highly reactive molecule that is produced by
NOS. It plays a fundamental role in maintaining normal vasomotor
tone (15). As mentioned
previously in this discussion, mucosal blood flow is an important
defensive factor that protects the gastric mucosa. The level of NOS
expression in the gastric mucous membrane epithelial cells may, to
a certain extent, reflect gastric mucosal blood volume. EGF is a
crucial factor for healing in PUD. EGF and EGFR protein expression
is involved in the re-epithelialization and peptic ulcer healing
process, which is associated with the proliferation,
differentiation and migration of gastric mucous surface epithelial
cells (16). The histological
results of this study showed that the expression levels of the NOS,
EGF and EGFR proteins were not significantly different between the
high and low bleeding risk groups in the extreme hot or cold
climates. Despite this finding, when the associations between the
peptic ulcer-related factors and climate were studied, it was found
that the expression of occludin, NOS and EGFR was significantly
lower in the extreme cold climate than that in the extreme hot
climate. This result may explain why the incidence of peptic ulcers
increases in the winter and decreases in the summer, exhibiting a
clear seasonal pattern. However, it is likely that the seasonal
pattern of PUB is not associated with the expression of the NOS,
EGF and EGFR proteins in the gastric mucous membrane epithelial
cells.
HSPs are a set of proteins whose expression is
induced by heat shock and a number of other stresses. In recent
years, HSPs have been implicated in gastro-duodenal defense
mechanisms at the intracellular level. HSP70 is a type of HSP
induced by molecular chaperones, and it is involved in various
biological activities, such as rescue from apoptosis, protection
from cytotoxic damage, e.g. from NSAIDs or H. pylori
infection, and facilitation of ulcer healing (17,18).
The present results demonstrated that the expression of HSP70 in
the high bleeding risk group was significantly lower than that in
the low bleeding risk group in the extreme cold climate; however,
the difference was not significant in the extreme hot climate. In
addition, the expression of HSP70 was significantly lower in the
extreme cold climate than that in the extreme hot climate. The
present findings suggest that low expression of HSP70 in the
gastric mucosa may play a key role in the mechanism of PUB in
extreme cold climates.
Mucus is a major gastric mucosal defensive factor
that functions at the first level of the gastro-duodenal defense
system. In the present study it was observed that the gastric mucus
thickness in the high bleeding risk group was significantly lower
than that in the low bleeding risk group in the extreme cold
climate. This reduction in thickness may weaken the gastro-duodenal
mucosal defensive mechanism.
Previous studies have demonstrated that PUD has a
seasonal pattern, but it has been unclear whether climatic factors
also influence PUB (19,20). The results of the present study
indicate that PUB induced by extreme cold climate may be associated
with low expression of HSP70 and a reduction in gastric mucus
thickness. There is substantial evidence that HSP70 plays an
important role in the mechanisms of PUB (21). We hypothesize that extreme cold
climate disrupts the barrier factors of the gastric mucosa, thereby
permitting aggressive factors to damage the gastro-duodenal
epithelium, which may progress to PUB. In the present study, the
expression levels of the NOS, EGF and EGFR proteins were not
significantly different between the high and low bleeding risk
groups in the extreme climates; however, a seasonal fluctuation in
PUB was observed. Although the rate of H. pylori infection
between the high and low bleeding risk groups was statistically
insignificant in this study, H. pylori infection is the most
common pathogen associated with PUD and PUB. In conclusion, the
decrease in barrier factors and increase in damage in the extreme
cold climate may explain the pathogenesis of PUB in extreme cold
conditions. Therefore, enhancing the gastro-duodenal mucosal
barrier system could aid in preventing PUB in extreme cold
climates.
Acknowledgements
This study was supported in part by the Key Project
of the National Research Program of China (no. 30660067), the
Jiangxi Province Talent 555 Project, the National Science and
Technology Major Projects for ‘Major New Drugs Innovation and
Development’ of China (no. 2011ZX09302-007-03), the National
Natural Science Foundation of China (no. 81470832), the Doctoral
Scientific Fund Project of the Ministry of Education of China (no.
20123601110003) and the Graduate Innovation fund of Jiangxi
Province (no. YC2014-B020). The authors would like to thank
Medjaden Bioscience Ltd. for assisting in the preparation of this
manuscript.
References
|
1
|
Vucelić B, Milicić D, Ostojić R, et al:
The effect of atmospheric conditions on the occurrence of peptic
ulcer hemorrhage. Lijec Vjesn. 115:70–73. 1993.(In Croatian).
|
|
2
|
Boles RS and Westerman MP: Seasonal
incidence and precipitating causes of hemorrhage from peptic ulcer.
J Am Med Assoc. 156:1379–1383. 1954. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bekku D, Arai M, Imazeki F, et al:
Long-term follow-up of patients with hepatitis B e antigen negative
chronic hepatitis B. J Gastroenterol Hepatol. 26:122–128. 2011.
View Article : Google Scholar
|
|
4
|
Yuan Y, Padol IT and Hunt RH: Peptic ulcer
disease today. Nat Clin Pract Gastroenterol Hepatol. 3:80–89. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Laine L, Takeuchi K and Tarnawski A:
Gastric mucosal defense and cytoprotection: bench to bedside.
Gastroenterology. 135:41–60. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Fatović-Ferenčić S and Banić M: No acid,
no ulcer: Dragutin (Carl) Schwarz (1868–1917), the man ahead of his
time. Dig Dis. 29:507–510. 2011. View Article : Google Scholar
|
|
7
|
Hermansson M, Ekedahl A, Ranstam J and
Zilling T: Decreasing incidence of peptic ulcer complications after
the introduction of the proton pump inhibitors, a study of the
Swedish population from 1974–2002. BMC Gastroenterol. 9:252009.
View Article : Google Scholar
|
|
8
|
Gralnek IM, Barkun AN and Bardou M:
Management of acute bleeding from a peptic ulcer. N Engl J Med.
359:928–937. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Axon A: Helicobacter pylori and Public
Health. Helicobacter. 19:68–73. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Henriksson AE, Edman AC, Nilsson I, et al:
Helicobacter pylori and the relation to other risk factors in
patients with acute bleeding peptic ulcer. Scand J Gastroenterol.
33:1030–1033. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Matsukura N, Onda M, Tokunaga A, et al:
Role of Helicobacter pylori infection in perforation of peptic
ulcer: an age- and gender-matched case-control study. J Clin
Gastroenterol. 25(Suppl 1): S235–S239. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Okan A, Tankurt E, Aslan BU, et al:
Relationship between non-steroidal anti-inflammatory drug use and
Helicobacter pylori infection in bleeding or uncomplicated peptic
ulcers: A case-control study. J Gastroenterol Hepatol. 18:18–25.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Gladwin MT, Crawford JH and Patel RP: The
biochemistry of nitric oxide, nitrite, and hemoglobin: role in
blood flow regulation. Free Radic Biol Med. 36:707–717. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Park T and Wassef W: Nonvariceal upper
gastrointestinal bleeding. Curr Opin Gastroenterol. Sep
16–2014.(Epub ahead of print). View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chang SS: Helicobacter pylori Eradication
within 120 Days was associated with decreased complicated recurrent
peptic ulcers in peptic ulcer bleeding patients. Gut Liver. Jun
18–2014.(Epub ahead of print). View
Article : Google Scholar
|
|
16
|
Syam AF, Sadikin M, Wanandi SI and Rani
AA: Molecular mechanism on healing process of peptic ulcer. Acta
Med Indones. 41:95–98. 2009.PubMed/NCBI
|
|
17
|
Choi SR, Lee SA, Kim YJ, et al: Role of
heat shock proteins in gastric inflammation and ulcer healing. J
Physiol Pharmacol. 60(Suppl 7): 5–17. 2009.
|
|
18
|
Tarnawski AS, Ahluwalia A and Jones MK:
The mechanisms of gastric mucosal injury: focus on microvascular
endothelium as a key target. Curr Med Chem. 19:4–15. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Fares A: Global patterns of seasonal
variation in gastrointestinal diseases. J Postgrad Med. 59:203–207.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Liu D, Gao A, Tang G and Yang W: Study of
the relationship between the onset of peptic ulcers and
meteorological factors. Chin Med J (Engl). 116:1940–1942. 2003.
|
|
21
|
Mizushima T: Protective role of HSP70
against various gastrointestinal diseases. Curr Opin Pharmacol.
19C:1–5. 2014. View Article : Google Scholar : PubMed/NCBI
|